46. Everything in Focus Remains Unclear
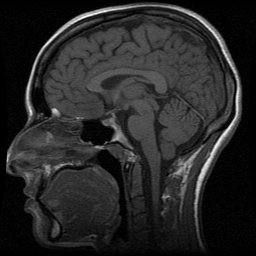
A neurologist orders my first magnetic resonance imaging (MRI) scan in 2007, based on my history of headaches, facial numbness and tingling, visual snow, and visual hallucinations. Sagittal and axial T1 pictures of the brain are taken to evaluate for the presence of anomalies ranging from sinusitis to tumors. The sagittal pictures in particular resemble every brain MRI I have seen on TV, crisp and grayscale, with black tar pits and canals for my sinus cavity, gustatory caverns, and spaces in the brain, and the fatty tissue of the scalp outlining my head in white. Sans contrast, it's the slice that looks most like an autopsy photograph or a post-mortem dissection halving the organ like a hedge apple. The interpretation, signed by three radiologists and reviewed by Nancy before being released to me, affirms a diagnosis of "labyrinthitis nos [not otherwise specified]," or inflammation of the inner ear and/or cranial nerve VIII, but the notes characterize the organ as normal: "The ventricles and cortical sulci are normal in size. There is no region of signal abnormality in the brain parenchyma. There is no area of restricted diffusion to indicate a recent infarct. No mass effect or extra-axial collection is seen. The cerebellar tonsils are in normal position [emphasis mine]. Normal flow voids are seen within the internal carotid and basilar arteries."
The membranous labyrinth and vestibulocochlear nerve are not mentioned. During the process of medical decision-making deliberations that precede a sign-off, these radiologists must have seen something, maybe something so obvious that for them it needs no recording. My inexpert eyes stutter on the (my) patchy shadows at the top of the skull, on the (my) gray cerebellar tonsils, which appear to descend ever so slightly below the base of the skull. There is nothing in the notes about that, either. Uninitiated into the mysteries of radiology, I assume I am wrong.
I know I can't see pain here, but instinctively, I look.
All looking is directed (Stafford, 1993; van Dijck, 2005; Segal, 2005; Teston, 2004). Visualizing the opaque body in the biomedical complex exceeds a one-to-one representation of reality; in Teston's (2004) words, the visual makes meaning-making and the construction of a reality possible. Laboratories and radiological departments capture and present visible facts, which clinics, through rhetorical acts of interpretation and diagnosis, use to establish norms (Mol, 2002, p. 123). If previous images exist, side-by-side comparisons are made. Radiological and pathological information, along with the patient's background and medical history, are discussed. Medical images permit clinicians to view disease in the larger context of the body, affording deliberators the opportunity to move towards evidence-based decisions, to stage the disease and determine both prognosis and the level of aggression in treatment (Teston, 2004, pp. 200-202). That said, as Alač and Hutchins (2004) observe, "brain images do not function as self-explanatory representations that simply support scientific reasoning and practices. Despite the fact that MRI measurements are made visible, a great deal of interpretive work is required to render the visible images meaningful" (p. 632).
MRI technology produces digital cross-sections of the body using magnetic fields instead of x-rays, rendering it capable of seeing through bone without the aid of a scalpel or saw (van Dijck, 2005, p. 6). During a brain MRI, hydrogen protons in brain tissue are induced to emit signals that are read by the computer, translated into numerical data, and converted into a neurological portrait (Alač & Hutchins, 2004, p. 631). On a standard MRI, air and moving fluid, such as blood in an artery, appear black, and soft tissues are grayscale, varying in gradation based on their water and fat content as well as the machine settings and whether or not gadolinium contrast media is present. While functional MRI (fMRI) scans capture dynamic images of brain functions by visualizing alterations in magnetic field properties in the brain, the static MRI is a detailed photograph of brain morphology, sensitive enough to detect stroke, epidural hematoma, abscesses, inflammation, acoustic neuromas, Chiari's, and other structural anomalies. Medically and socially, wellness is associated with normative morphology; personality is correlated with brain activity and blood flow. In the same way that the transvaginal ultrasound has come to evoke pregnancy and motherhood, the brain MRI connotes the threat of brain cancer, mental illness, cognitive defect.
In addition to identifying signs of neurological disorder, neuroimaging promises to render emotion and intelligence objective, visible, and confirmable. Of all of my medical images, my brain MRIs are most like a mirror, whose deceitful reflection promises: I am a marvel of clean outlines and symmetry; I work.
Medical imaging contributes to the social construction of disease and its cure by visualizing it in situ (van Dijck, 2005, p. 12). Crary (1996) suggests that new visualizing technologies contributed to a cultural reconfiguration of the relations between the observer and modes of representation: like other imaging technologies, the MRI is a product of technological coevolution, emerging alongside advancements in computing. According to Dumit (2004) and Graham (2009), imaging technologies are often legitimized-by-metaphor in the courts and mass media through photographic and cartographic analogies; in these analogies, they are typically "black boxed," the process by which the complexities of a technology are smoothed over while the technology is authorized (Graham, 2009, p. 387). New technologies borrow the black boxes of their black-boxed predecessors: for instance, PET is legitimized in reference to CT, which is legitimized in reference to MRI, which is legitimized in reference to x-ray technology, which is legitimized in reference to photography, which refers to cartography, which appeals to eyesight, the final arbiter of epistemology in the Euro-Western sensorium (Dumit, 2004, p. 114; Graham, 2009, p. 388; Arnheim, 1969; Jay, 1993).
Radiologists select and view based on the black boxes and visual codes that have come before, but to the patient, radiological images are mystified; they belong to the clinical domain. Nevertheless, chronically ill patients who have necessarily seen many medical images of their bodies over time often learn how to identify the key radiological features of their own organs and structures (Howell, 1995, p. 161). These technologies and the hermeneutics that govern them remain relatively black boxed to me, but I can locate my limbic system, my cerebellum, my optic nerve, places that might be responsible for or involved in chronic painervation. I can't, however, locate pain.
MRI technologies have shown that there is no single specific pain center in the brain (Martucci et al., 2014, pp. 621-622). MRI and fMRI have been used to track changes in white and gray matter, with attention paid to atrophy, changes in density, and blood oxygenation. Neuroimaging research on chronic pain uses MRI and fMRI to study altered activity levels in the central nervous system, where pain modulation and central sensitization occur. These forms of neuroimaging are perhaps the most widely used biomedical technologies for studying nociception in fibromyalgic patients, as they permit the study of patients at rest or engaged in tasks designed to stimulate regions of the brain, such as viewing emotionally evocative images or enduring pressure or heat stimuli (p. 616). In an fMRI study, Gracely and Ambrose (2011) find that fibromyalgics produced cerebral responses to pressure that were qualitatively and quantitatively twice that of control subjects, and that they use more brain resources than control subjects to accomplish cognitive tasks (pp. 278, 281-282). And PET data was instrumental in the first major Lyrica clinical trial for fibromyalgic sufferers, indicative of the role of neuroimaging in ontologizing fibromyalgia (Graham, 2009, p. 398).
My clinical neuroimaging procedures involved standard MRIs, but I received an fMRI in a research study at Columbia University's Functional MRI Research Center, maybe in 2008, in which I was shown images of emotional states on a projector screen to identify forms of neural activity and where they take place. I didn't see the pictures, and I don't know the results, but in the dark tunnel illuminated only by that screen, I wondered if I was skewing the data, because the photographs of faces plastered in grimaces or thousand-yard stares evoked friendly recognition in me, not alarm.
In any case, this 2007 diagnostic imaging exam was not ordered to locate pain, but to rule out tumors, which the referring neurologist had already decided was the cause of my symptoms. Medical science has struggled to visibilize fibromyalgia, as muscle and joint deterioration do not reliably coalesce on x-rays or MRIs, and myofascial pain similarly elides technologies of visual capture (Barker, 2005, p. 4). I am told the procedure is painless, but lying still on the MRI couch for an hour is an agony that the camera doesn't catch. I endure because these images are supposed to translate my subjective reality, pain, into biomedical certainty by photographing incontrovertible etiological evidence, such as lesions, masses, crowding. As with the x-rays, however, the MRI results are normal, and I am left feeling like the conclusion of labyrinthitis nos — with no explanation offered by the interpreting radiologist in the notes — is a last-ditch maneuver to demonstrate expertise. It must be something, after all.
Inconclusive images that nevertheless are labeled with a diagnosis trap me between my symptomatic certainty and biomedical disbelief, a chasm that widens with every negative test (Barker, 2005). "Facts usually imply relationships between things that are not bound to time and space and culture; they simply are. Facts are not untethered, however; facts are facts-in-the-world" (Dumit, 2004, p. 159), and when we receive facts about our bodies, we are continually balancing the status and relative worth of these facts, in various settings and for ourselves. In 2007, I possess the fact of myself as neurologically unremarkable. In 2016, an MRI scan that produces similar images is recorded as "a normal examination," but it includes this curious phrase: "The cerebellar tonsils lie below the foramen magnum, a normal variant." I ask Dr. Kyrios for a comparison of both my 2007 and 2016 images, talking around Chiari malformation without naming it, and she tells me that the low lying cerebellar tonsils were apparent in both sets, but they aren't compressed or low lying enough to need a surgical evaluation. Low lying is code for less than 3mm, which is allegedly benign and asymptomatic. I have heard anecdotally that even within the normal range, symptoms like pain and poor balance can occur.
I think back to my kneejerk reaction in 2007, and I feel justified, but not much else. 2016 marks a decade of armchair radiological study, carefully scripted medical pleas, cautious and deferential requests for medical explanations. I am too depleted to push.
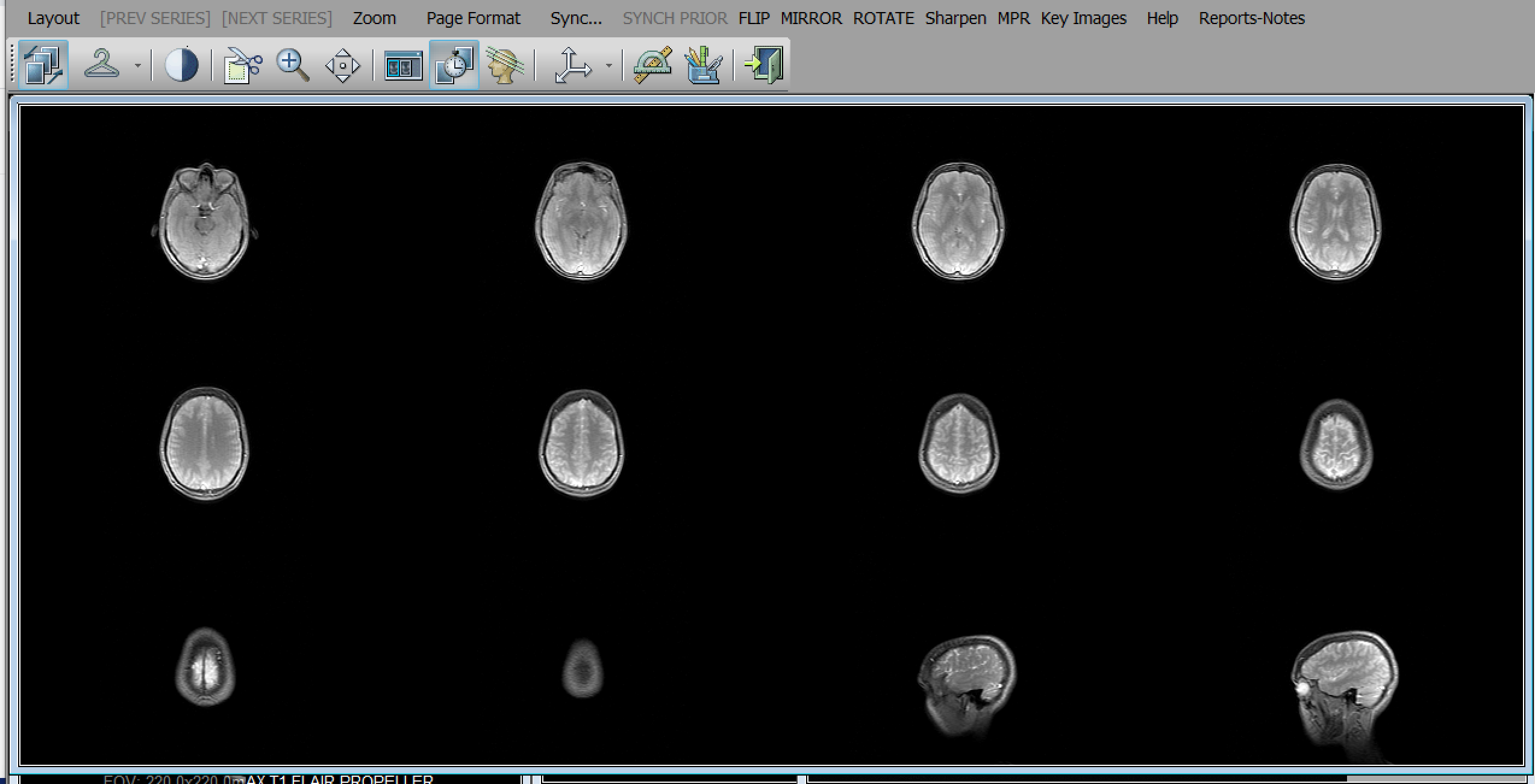
In order to view both the 2007 and 2016 images, I must use a Digital Imaging and Communications in Medicine (DICOM) viewer, software that is a puzzle in its own right but can't be separated from the images it displays. With the proliferation and advancement of biomedical imaging technologies, establishing a network standard became increasingly essential. The DICOM standard controls the input and output of medical imaging system data, so that equipment properly designed and configured to DICOM specifications will be able to reliably communicate with other equipment operated through DICOM interface (Bidgood et al., 1997, para. 1). The DICOM protocol is compatible with TCP and IP, enabling DICOM equipment to communicate online. Even a full-screen display does not hide the graphical user interface, which features non-intuitive icons alongside recognizable playback controls: a coat hanger, a stack of books, parallel diagonal lines. The technology is not transparent, not any more than my pictured body is. It reminds me of the journey my images have made: first, the MRI scanner initiates the image transfer during the procedure, or the operator activates a "send images" function on the scanner console after all the images have been acquired; the pictures are sent to the workstation; the scanner initiates a DICOM association for each image; and the scans are sorted by explicit semantics enabled by DICOM, so images may be retrieved through an indexing system based on clinical relevance and image attributes (paras. 4-12).
X-rays, MRIs, CTs, ultrasounds, radiofluoroscopy, and other diagnostic imaging modalities conform to DICOM protocol. Standardization means my scans are easily shared among the members of my medical team, and the DICOM format, which has no set window level or width, makes adjustment and postprocessing easier for radiologists, but it also serves a gatekeeping function. I can't intuitively navigate my own images. I struggle to export picture and video files, alter the frame rate, add or delete annotations or reference lines that show my untrained eyes nothing. My clumsy clicking around generates arrangements, magnifications, and luminance I don't want. I grant fictive motion to static images by scanning my gaze over them and click-and-drag panning through the frames. I am presented with machinic atlases, which train the eye to seclude certain objects as exemplary and disregard others as uninformative (Daston & Galison, 1992, p. 85).
The interface emphasizes the machinic nature of these computerized images, heightening their aura of objectivity. According to Dumit (2004), "brain imaging's power comes to be a combination of scientific and medical authority, machinic and now digital objectivity, as well as cultural norms and social desirability. In many cases, ironically, this persuasiveness comes to exceed the authority and even ability of the image's authors: The very experts who made them can no longer delimit what they mean" (p. 113). In other words, medical images are persuasive outside the clinic when read by lay audiences, such as juries in courtrooms, fans of televised medical dramas, or even gallerygoers. I see a sheet of miniature portraits, vaguely reminiscent of a Warhol repetition in their arrangement, apparitions brightening in the darkness, glowing as though lit from within. This brain, my brain, recognizably me in sagittal profile, betrays me with its ordinariness. I know it is haunted. I know from the reports, in 2007 and 2016, it is normal. I know in 2020 that a reactivated virus likely took up residence in the limbic system and hippocampus, back in 2006 when all of this began.
Question: What does my pathology look like?
Answer: Like a pey.
According to Dolphin-Krute (2017), "a medical image means nothing until it is determined what, exactly, it does mean, which is not the tautology it seems like. It is more like saying the MRI image exists in two parts: as an image, and then as an image conveying specific information. The processes or ordering of attaching and detaching information and image will depend on the specific viewer, the specific body, the specific interpretation at hand" (p. 34). Interpretations are subjective and co-constructed through shared images and boardroom deliberations. Specialists like the white male neurologist Nancy refers me to and Dr. Kyrios, recommend and order diagnostic MRI exams. The scans are performed on DICOM equipment, the images shared between machines, workstations, and radiologists. The machine operator, who selects what to photograph, sees them. A radiological team sees them. Since MRIs can produce hundreds of images, key images — images that are clinically significant — are flagged in DICOM. Their meaning might be debated. A radiologist writes the interpretation and signs it, becoming the visible, expert author of the verdict (Teston, 2017). The ordering physicians — in my case, the white male neurologist and Dr. Kyrios — paraphrase the MRI results in a manner that naturally underscores what they find important. From this, I distill what I think is important — that is, what seems to reflect my embodied experience and symptomatology — as I filter the images and reports through my understanding of medical information about my specific body.
These experts speak for the laboratory and the neuroscience community, situating this way of seeing in collective empiricism and scientific cultural knowledge. Consequently, if a patient wants to challenge their physician's opinion, they are tacitly going up against an entire scientific field, a much more arduous task with ominous repercussions for future treatment (Daston & Galison, 1992; Alač & Hutchins, 2004).
These pictures are of my specific body, and I feel a paradoxical estrangement from and ownership over them: my brain, my ovarian cyst, my ruptured appendix. The parts that seem morphologically concerning to me, however — my fifth cranial nerve (CN V), or my cerebellar tonsils — are not noted as suspicious. This despite the fact that Dr. Kyrios later confirms a diagnosis of trigeminal neuralgia. Despite the fact that laboratory tests and microscopy point to reactivated HHV-6, the virus that likely caused my chronic fatigue and that often cozies up to the central nervous system. Despite the fact that later in 2007, I see an ENT specialist about labyrinthitis, and she flushes my ears of wax and shines a light into each and escorts me to a soundproofed booth for an audiometry test, and she tells me that whatever is wrong with me, it's nothing to do with my ears.
Everyone has a different idea of importance. Radiologists drone on about morphology while I wait with bated breath for the ghost story.
Dolphin-Krute (2017) likens medical imaging to portraiture, saying, "An MRI is inherently an image of a living body. It is not an autopsy photograph. It is, as a photograph, an unintelligible living record and for that reason a portrait form fitting for ghosts" (p. 32). Unlike portraits, however, sensory information about the setting is omitted. You can't see the white tunnel, the lighting, the blue paper gown; you can't see my brown skin, my butch hair, my multiple earring holes, my face contorted with the strain of remaining motionless (pp. 32-35, 40). The cultural and visual logics of the diagnostic image align personhood (and rehabilitation) with the technological image and biological sample, not with the sensing, sense-able body being imaged (Dumit, 2004; van Dijck, 2005). I can't sense my brain sensing. Paradoxically, although it modulates visceral function, sensory experience, voluntary movement, embodied thought, it is inaccessible to my conscious apprehension, bastioned within the skull, utterly withdrawn from the world (Leder, 1990, pp. 113-114). The brain is absent to perception and interoception, yet the MRI visually captures what interoception cannot perceive, without inscribing any sense of sensing in the image. When I open my abdominal CTs in the DICOM viewer, I reflexively correlate the kaleidoscoping of my viscera with the pain of peristalsis, the bulge and scrape of moving feces, the sharp unkinking of the Mariana Trench of scars bridging my hips. When I view my brain, I know it is mine, I perceive it and make meaning with the very same brain, but I have no sensations to ascribe to the image. Like viewing myself autopsied, a specimen in formaldehyde in an anatomy lab.
Stripped of gender, race, sexuality, and even disability in the image, I tell myself I'm recognizable in profile. With my creative background, portraiture is how I apprehend my imaging scans, particularly when I'm not sure how else to read the image. The sagittal view is a crisp image in a medical textbook displaying the inner workings of my brain. The top view looks like its gestating life, a Rorschach blot, or the torso of a woman, princess Kunti throwing open her window to greet Surya, the god's crest seared into the sky above her. The axial view, like an evil eye set in a rapacious yaksha's face, staring out of a gnarled tree or crumbling stone. The 4x3 layouts of miniature brain portraits, like the photo booth strips in Japan where we'd crowd in and flash victory signs. These are all my head and I am not in it. A haunting at the margins. A presence enveloped in the blackness of the border.
Like the x-ray before it, MRI technology radiates authority and aspires to infallibility, and our belief in it, bolstered by pop culture representations and its courtroom uses, guarantees its authenticity (Daston & Galison, 1992, p. 116). It is an ideal observer, representing the body with impeccable fidelity, and I am an Eelam Tamil woman who frequently loses her battles with the medical system, so I will not say the machine lies, but it does not believe in ghosts; therefore, it does not believe in me.
"If a ghost is an image of a person without a body, then an MRI, rendered in ghostly gray scale, is an image of a body without a person" (Dolphin-Krute, 2017, p. 32). If the body without a person is also a body with a non-apparent chronic illness, if the person who owns the non-apparently chronically ill body is already a ghost, then an MRI, which does not render it, is a snapshot of a double haunting.
In 2007, I say I trust the images, and I progress to the next stage of diagnosis. In 2016, though I like Dr. Kyrios, who has treated me fairly and intelligently, I don't ask if low-lying cerebellar tonsils are ever symptomatic. I don't trust her enough for that. Being chronically ill in academia and the biomedical complex has taught me, everyone only goes so far. The image renders a rhetorical construction of illness or wellness in diagnosis, prognosis, and treatment (Teston, 2017, p. 190). To miss the ghost is to mistreat the patient. To stare directly at a ghost risks pey pidichittu. Few medical professionals wants to risk possession, and you don't sustain scientific credibility by listening to ghost stories told in mixed-metaphors and brain-fog blends.
I am witnessing these failures of expertise all the time. The more you try to conjure a figure into view, the more it fades away.
(– 86. There Are No Words for This But Here Are Mine)